Chapter 2 Life’s secrets for storing energy
Chapter summary:
- There are five major requirements for life to happen on our planet, which include liquid water, CHONSP atoms as nutrients, a source of energy, electron acceptors, and suitable temperatures
- Living organisms have found ways to store energy in the form of electrons on the C, N, and S atoms
- This energy can be released through electron transfer to electron acceptors
- Oxygen, because of its high electronegativity and its assemblage into O2, tends to ‘steal’ electrons from other atoms and drives electron transfer and energy generation
- Electronagitivity is a powerful tool to visualize where and how many electrons are stored on C, N, and S atoms in inorganic and organic molecules
Much of the environmental and ecological engineering challenges are about dealing with too many suspended sediments and too many nutrients in surface and ground waters, and the consequences of too much organic matter and reduced compounds in aquatic ecosystems. We have also seen that these three challenges are inter-connected and related. Nutrients and organic matter are related because the former contain the needed atoms necessary to build living cell and organism structures, corresponding to living organic matter, while the latter generally refers to dead organic matter and/or the assemblage of molecules which at one point were part of a living organism.
This definition of the linkage between the two is hardly satisfying, however… When one looks at where the electrons are allocated on the important atoms, a much more unifying scheme appears. This chapter describes this unifying scheme.
2.1 The five fundamental requirements of life
Before we dive down to the electron level, let us make sure we recognize that it is possible to simplify why there is, or not, life on our planet and the universe. At least five requirements appear fundamental for life to exist on our plant. Such a list includes:
- the presence of liquid water
- available nutrients that can fulfill the need for the six most numerous and important atoms that build most of our cell and organism structure: C, H, O, N, S, P. Without the commas comes this delightful acronym CHONSP which generations of students have come to love
- a source of energy, which in most cases is in the form of solar or chemical energy
- electrons acceptor(s), without which chemical energy cannot be released
- a suitable temperature range (about -5°C to +50°C) because of course most of living organisms will not live for very long outside this range
There are many other requirements for life to occur, e.g., the ability to reproduce or even to die, but for what we are interested in, this is a satisfying list. In this chapter, we will address sources of energy and the secret that living organisms have found to store energy. Primary producers, which include most of chlorophyll containing plants from algae to angiosperms (flowering plants), have the ability to use solar radiations as a source of energy. An entire chapter is dedicated to this marvel.
However, solar radiation cannot be the sole source of energy for primary producers, otherwise they would die at night… And for the rest of living organisms, solar radiation is just not a source of energy (merely a source of ‘bien-être’ or vitamin D for humans…!). So obviously, life has had to find a solution so that energy would be available for all conditions of light and temperature on earth.
The first secret of life is the ability to store energy in the form of high energy electrons stored within organic molecules. Having a source of energy is always a good thing. However, energy can only be released if there is an outlet for it, otherwise it stays as potential energy. Practically, this means that the energy stored on organic molecules can and is only released when the electrons go from the high potential to a lower one, or in others words from electron donors (most of the time from organic molecules) to electron acceptors.
This is the second secret of life on earth: on our planet exists this miracle molecule, O2, which acts as an extremely powerful electron acceptor. So you have to imagine O2 less as a gentle acceptor of electrons that organic matter would be kind enough to donate, but more like a very aggressive electron seeker and any organic matter located close to oxygen runs the risk to be oxidized, that is to lose its electrons. Sometimes, I like to refer to O2 as the electron kleptomaniac. So in oxygenated environments such as earth’s atmosphere and most water bodies, living organisms’ only concern is to have potential energy available in the form of high energy electrons stored onto organic molecules, because this energy can easily be released at any time thanks to the very oxidizing agent O2. Chapter 5 goes into the details of how this energy is released and transferred in cells.
2.2 Electron allocation onto CHONSP
First, among the 6 atoms that form CHONSP, and this is true for all atoms except for noble gases which are stable monatomic atoms, none of them exist as monatomic atoms: they always form bonds with other atoms to form molecules. Among the CHONSP, three of them are homonuclear diatomic molecules, i.e., they can form molecules of two atoms of the same chemical element: H2 (dihydrogen, although it is most often referred to simply as hydrogen, which can be quite confusing), O2 (dioxygen, although oxygen is also most often used, unfortunately), and N2 (dinitrogen, which name is generally properly applied). Obviously, they can also bind to other elements, which is what we are about to see.
If indeed, on our planet floats a very oxidizing agent in the form of O2, then the stable state of all other molecules should be where most of their elements have lost all their electrons to oxygen. And there ought to be techniques to see where the electrons are allocated on molecules.
2.3 Electronegativity as a powerful tool to allocate electrons
Chemists have created the oxidation state (OS) or oxidation number indicator which quantifies this electron allocation to some extent, but at this stage, we find it to be rather confusing for our purpose of allocating or view where the electrons are Stored in molecules. More discussion and information on Oxidation State is available in the glossary of this book. Oxidation States are used extensively later on.
We must first look in the electronegativity concept. Electronegativity is a chemical property which describes how well an atom can attract an electron to itself. In the periodic table in Figure 2.1, first observe that our CHONSP all belong to the non-metallic atoms.
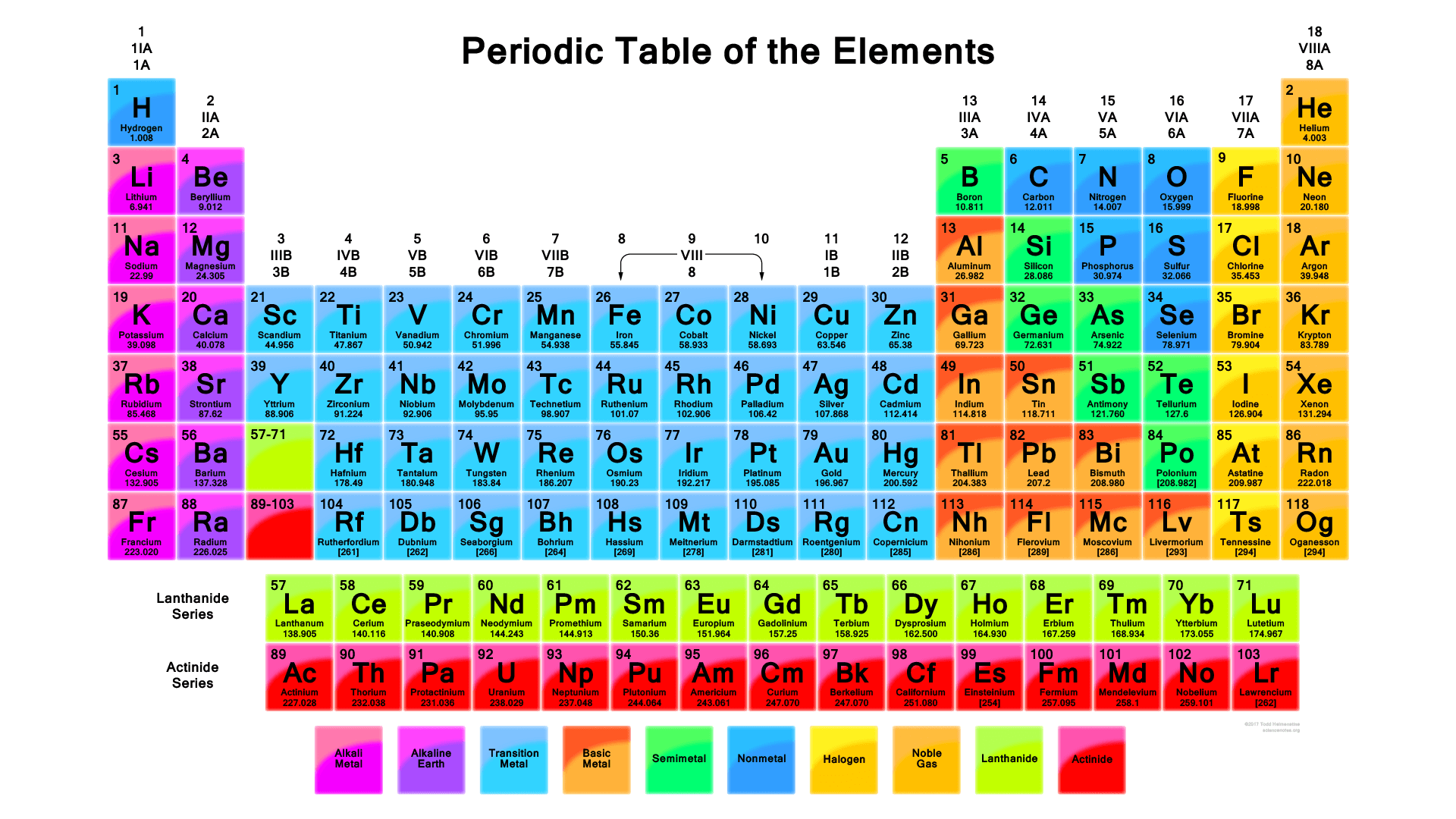
Figure 2.1: Vibrant Periodic Table With 118 Elements. Obtained freely from https://sciencenotes.org/periodic-table-pdf-2/
Second, all atoms are more or less electronegative. The most electronegative atom is F, fluorine. Electronegativity decreases towards the left and the bottom of the table as illustrated in Figure 2.2. For our CHONSP, ElecNegO > ElecNegN > ElecNegS > ElecNegC > ElecNegH > ElecNegP.
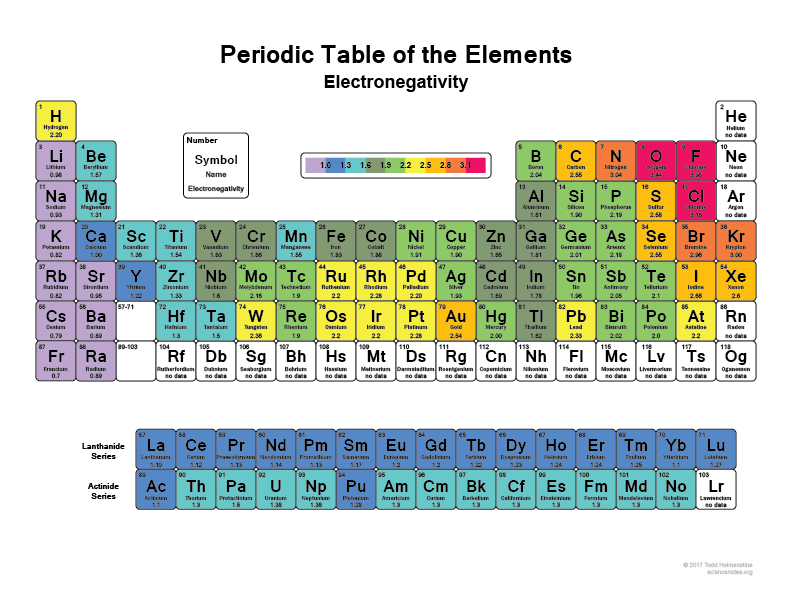
Figure 2.2: Periodic table of the elements with electronegativities shown. Obtained freely from https://sciencenotes.org/list-of-electronegativity-values-of-the-elements/
If you look carefully in Figure 2.2 and in equation (2.1), you will see that the electronegativity of oxygen is 3.44, which is a lot greater than that of N (3.04), and the difference between N and C and S, is about 0.5. In other words, all the CHNSP stand no chance next to O: in any molecule which may involve CHNSP, the O atom will attract the electrons to itself.
\[\begin{align} O \quad &>& N \quad &>& S \quad &>& C \quad &>& H \quad &>& P \\ 3.44 \quad &>& 3.05 \quad &>& 2.58 \quad &>& 2.56 \quad &>& 2.30 \quad &>& 2.19 \tag{2.1} \end{align}\]
The electronegativity numbers give an estimation of how much the electrons will be pulled towards the more electronegative atom compared to the less electronegative one, sometimes creating some polarization of the molecule. Here we use this concept a little further and allocate all the electrons to the more electronegative atom. This is admitedly artificial and, again, in reality electrons are shared and electronegativity only describes the tendency of an atom to attract a shared pair of electrons towards itself. However, allocating electrons using the electronegativity as a basis is an extremely powerful tool to visualize where electrons are and whether and how much energy is stored on organic matter.
For example, in the H2O molecule, one can see in the Lewis dot Structure below that by allocating the electrons based on electronegativity, the O atom has all the electrons for itself, while the 2 H, have none for themselves. Let us make sure that there is no confusion between the octet rule and the electronegativity as applied here. Atoms assemble into molecules so that their octet rules be fulfilled. So in the H2O molecule, yes each H shares an electron with the O and reciprocally. But, allocating the electrons using the electronegativity as detailed above tells us which atom really has the electrons for itself. And that atom is O.
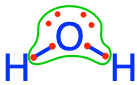
Figure 2.3: Electrons allocated to the oxygen atom on the water molecule using electronegativity as a guide for allocation. This shows that oxygen keeps all 8 electrons for itself, while the hydrogen atoms have lost their electron
We realize that the electron allocation can be quite confusing or a bit artificial at first, but we shall see in Chapter 8 that it is fully corroborated with oxidation and reduction processes. You can now see in equation (2.1), that sulfur and carbon nearly have the same electronegativity, which is one of the reasons for the ease of formation of the disulfur bridges in proteins. Also notice that phosphorus has the lowest electronegativity of our CHONSP, and this is the reason why, as we shall see later, phosphorus stays in the oxidized form, even in organic molecules.
2.4 Dioxygen: the ‘electron kleptomaniac’ molecule
Oxygen is a homonuclear diatomic molecule, which means that it can form the diatomic molecule O2. The two atoms of Oxygen, having the same electronegativity, split the electrons, which means that each atom has 6 electrons for itself as shown in Figure 2.4. So if we summarize things, we have the most electronegative atom in the universe after Fluorine (Figure 2.2) that is floating in most places on earth and in water, essentially in a state (O2) where it really would rather have 8 rather than 6 electron for itself! This is the reason why oxygen is the ultimate electron acceptor, although a better representation might be to describe it as the ultimate ‘electron kleptomaniac’.
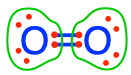
Figure 2.4: Electrons equally allocated to each O atom on the dioxygen molecule, i.e., each oxygen atom has 6 electrons for itself
But let us be clear: the oxygen atom can only be an electron acceptor if it does not have 8 electrons for itself. The only two possibilities are
- in the case of O2, for which each atom as 6 electrons for itself,
- and the other possibility is for hydrogen peroxide \(H-O-O-H\), where each oxygen atom have 7 electrons for themselves.
Because oxygen is the most electronegative atom (after fluorine), in all other molecules where it is involved, oxygen will have 8 electrons for itself, and can therefore never accept more electrons. This is the first time that this concept is presented in this book, but not the last, and the earlier the reader fully digests this information, the better. Again, and I purposely repeat here, in all other molecules where the oxygen atom is involved, oxygen will have 8 electrons for itself, and can therefore never accept more electrons. As a result, in organic molecules, the electrons allocated to oxygen are never available to be donated and should never be counted as stored electrons.
2.5 Fully Oxidized forms of CNSP
On our planet, we are lucky to ‘bathe’ in an oxidized environment, which means among other things, that the oxygen atom will tend to attract all the electrons for itself during interaction with CHNSP. Another way to look at it is to say that the stable state in an aerobic environment of the CHNSP atoms corresponds to molecules where the CHNSP atoms will have zero electrons for themselves (because oxygen stole them). In this case, we say that these atoms are fully oxidized, litterally, that oxygen has stolen all electrons from them. The action of losing electrons is referred to as oxidation by analogy. Reversely, an atom that has 8 electrons for itself is referred to as fully reduced, and the action of gaining electrons is called reduction. Although the terms were initially used to describe reactions with oxygen, losing or gaining electrons does not involve oxygen only, and the words oxidation (losing electrons) and reduction (gaining electrons) apply to any other atoms. Well, and guess what, there are not that many possible molecular configurations for which CNSP are fully oxidized, in fact only one for inorganic molecules with a single C, N, S, or P atom in them! In Figure 2.5 below, you can see that none of the CNSP atoms have electrons for themselves: they have all been taken by the oxygen atoms!
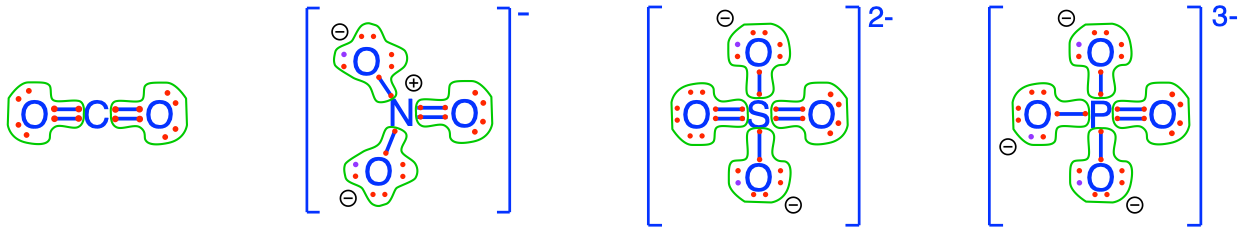
Figure 2.5: Electron allocation on each of the C, N, S, and P atoms for the CO2, nitrate, sulfate, and phosphate molecules, showing that C, N, S, and P atoms have zero electrons for themselves, or are fully oxidized, while Oxygen has 8, or is fully reduced
As a result, these molecules are deemed as stable in an aerobic environment. This suggests that all other molecules containing CNSP are essentially unstable on our planet…?! Yes, you read it correctly, that is the case! Honestly, there is nothing new really as this has been written a long time ago, e.g., in the first book of the Hebrew Bible or the Christian Old Testament “you are dust and to dust you will return” (Gn 3:19). So we essentially have a reprieve on this planet, but so do all living creatures!! The secret of life has been to be able to create and maintain CNSP atoms with electron for themselves, very well ‘knowing’ that these electrons would eventually be stripped away by O2, but living organisms take advantage of this because the transfer of electrons generates energy (see Chapter 5).
Notice that the suffix of the oxidized forms of N, S, and P, is -ate. The exception appears to be CO2, which is a gas while the -ate molecules are dissolved ions. However, the ionic forms of dissolved CO2 are bicarbonate and carbonate, which have the suffix -ate. So everything is logical in the end.
To be complete, all the fully oxidized forms of CNSP may lose or gain protons (H+) depending on the pH. It turns out that for the pH range encountered in most natural waters (3.5 < pH < 9), only the carbonates and the phosphates may have different molecular formulae. So the fully oxidized forms of C in inorganic molecules are illustrated in Figure 2.6 below (additional details available in the glossary):
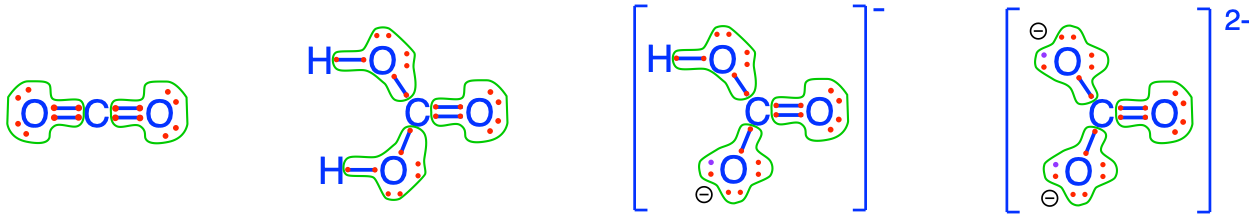
Figure 2.6: Electron allocation on each of the fully oxidized forms of C, including from left to right CO2, carbonic acid, hydrogen carbonate (or bicarbonate), and carbonate, showing that the C atoms have zero electrons for themselves
The fully oxidized forms of P in inorganic molecules are illustrated in Figure 2.7 below (additional details available in the glossary):
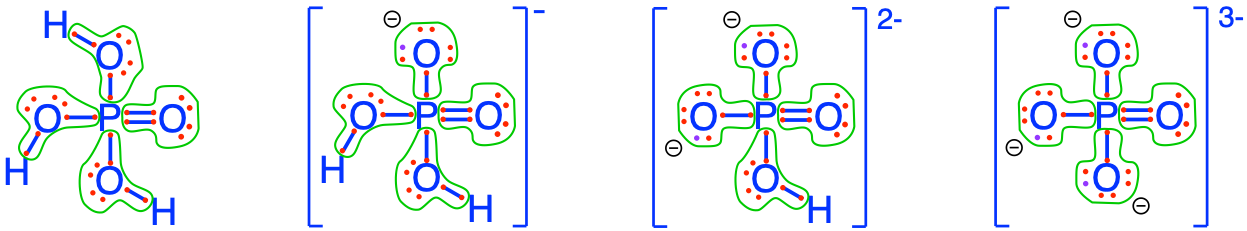
Figure 2.7: Electron allocation on each of the fully oxidized forms of P, including from left to right Phosphoric acid, dihydrogen Phosphate, hydrogen phosphate, and phosphate , showing that the P atoms have zero electrons for themselves
2.6 Oxidation versus Reduction
But if O2 wants electrons so badly from any atom, how come all living creatures do not just turn into ‘dust’, i.e., carbon dioxide, nitrate, sulfate and phosphate, all the time or manage to live at all?? A minimum ‘energy obstacle’ must be overcome for electrons to be transferred: this is referred to as the activation energy (see Chapter 5). This obstacle is overcome during combustion: the high temperature of a flame self-propagates the transfer of electrons from organic matter to O2. Chapter 5 provides in great detail how living organisms manage to allow enough transfer of electrons to generate energy, without turning into ‘dust’ at once like in combustion. Yet, when an organism dies, all the electrons stored onto the organic molecules are eventually taken by oxygen in an aerobic environment.
We have now established that oxygen eventually takes all electrons, but for that to happen, a minimum energy obstacle must be overcome. This opens the possibility for CNSP to, at least temporarily, have electrons for themselves. This also opens the possibility to an infinite number of possible combinations of CHONSP assemblages in nature.
Before we get there though, it is time to repeat some important terms. The word oxidation comes from the early days of chemistry referring to reactions where oxygen was involved, and in reality ‘stole’ electrons from other atoms. We now know that oxygen is not necessarily the only one involved in loss of electrons. By extention, any atom which loses electrons, not necessarily to O2, is said to be oxidized. Conversely, an atom which gains electrons is said to be reduced or to undergo reduction. This term also comes from the early days of chemistry when Antoine Lavoisier (1743–1794) observed that the weight of a heated metallic ore diminished due to the loss of dioxygen as a gas; and the metallic ore appeared reduced in size and in weight (Wikipedia contributors 2018f). We now know that this corresponds to the acquisition of electrons, hence the word reduction for the gain of electrons.
Several mnemonics exist in English to associate electron loss/gain with oxidation/reduction:
- LEO (Loss of Electrons is Oxidation) the lion says GER (Gain of Electrons is Reduction)!
- OIL RIG: Oxidation Is Loss (of electrons) Reduction Is Gain (of electrons)
When oxygen takes electrons from another atom, we say that oxygen has been reduced, while the other atom has been oxidized. An oxidation is necessarily accompanied with a reduction, as electrons do not ‘float’ in solution like protons do. When the CNSP atoms have lost all their electrons like in Figure 2.5, we say that the CNSP atoms are fully oxidized. Fully reduced atoms have 8 electrons for themselves, as is the case for oxygen in carbon dioxide (and carbonates), nitrate, sulfate, and phosphates.
This suggests that between fully oxidized and fully reduced, there might be cases where atoms are partially oxidized and reduced.
2.7 A gradient of oxidation and reduction states on inorganic and organic molecules
The number of electrons (1, 2, etc. up to 8) the CNSP atoms have for themselves dictates the atomic makeshift of molecules or molecular radicals. It is now time to define what organic vs. inorganic molecules are. The term organic molecule finds its roots in vitalism, where it was believed that the elements entering in the composition of living organisms had to be different to the ones that made inanimate things. The term organic has remained, and a more modern definition is that an organic molecule is a molecule where there is at least one \(C-C\) bond. The best definition of an inorganic molecule is a molecule that is not organic.
One of the most important things to understand and learn in this part is that it is the electron allocation which essentially defines what a molecule will be. For example, molecules such as carbon dioxide CO2 and methane CH4 are quite different, one involves oxygen, the other hydrogen atoms. But in reality, one can make sense of the difference by looking at how many electrons the C has for itself. When C has zero electrons for itself, this means that some other atoms have stolen all the electrons from C, and there are not that many possibilities out there. It could theoretically be all atoms that have higher electronegativity such as O,N, and S. And all three are theoretically possible. However, the only stable solution in an aerobic environment is O=C=O or CO2, carbon dioxide, and the carbonates (Figure 2.6).
For the record, among the other theoretical possibilities, there are other inorganic molecules that exist out there for which C has zero electrons for itself, which include at least: cyanate (N≡C-O-), carbon tetrachloride (CCl4), S=C=S (CS2 carbon disulfide) and O=C=S (OCS, carbonyl sulfide) (Carroll et al. 1986; Notholt et al. 2003; Campbell et al. 2008; Ogée et al. 2016), and also guanidinium (C(NH2)3+), all of which exist in minute quantities in nature. So, for environmental and ecological applications, it is fair to say that the ‘only’ carbon-based inorganic molecules with zero electrons are CO2 and the carbonates. Reversely, the only molecule containing one C atom with 8 electrons for itself is a molecule where all the other atoms bound to the C atom give their electrons away. The only atom that will do this is hydrogen, so the only possible molecule with a C atom is methane CH4. Thus, CO2 is an example of a molecule in which carbon is fully oxidized (lost all its electrons), while CH4 is a molecule in which carbon is fully reduced (gained all possible 8 electrons).
In other words, it is quite interesting to look at molecules from the stand point of the number of electrons atoms have for themselves and speculate on the different possibilities of molecules. Obviously, the examples of CO2 and CH4 are simplistic because they are simple, inorganic molecules. Two other simple examples but that of fully oxidized and reduced molecular “pairs” with relevance to matter very much in our field, are nitrate (NO3-) vs. ammonium (NH4+), and sulfate (SO42-) vs. hydrogen sulfide (H2S).
Identifying which of the molecules in the pair is more oxidized or reduced is simple. For fully oxidized compounds, the central atom of interest (e.g. S or N) must have no electrons to itself by definition. This electron deficiency must be caused by the presence of an atom more electronegative than the central atom. We might (correctly) guess then, that the fully oxidized form of S or N is attached to the “ultimate electron acceptor” oxygen. So, the fully oxidized versions of S and N are sulfate (SO42-) and nitrate (NO3-) respectively. Conversely, hydrogen-containing NH4+ and H2S are the fully reduced forms of N and S. This is because hydrogen, the least electronegative of CHONSP, “provides” electrons to N or S (Table 2.1).
Overall, across organic and inorganic molecules, there is an entire gradient of number of electrons allocated on the C, N, and S (Table 2.1). Notice that I purposely omitted to mention P here, because the only form of P relevant for environmental and ecological engineering are the phosphates. And we have seen that in the phosphate molecules, P always has zero electrons for itself. It is therefore always in an oxidized form (Figure 2.7).
Table 2.1 summarizes a gradient of electron allocations, or a gradient of oxidation and reduction states, on the CNSP atoms. It is important to notice several things:
- Carbon is the only atom with a variable number of electrons in organic molecules
- N and S always have 8 electrons for themselves in organic molecules
- This suggests that carbon is quite a variable of adjustment for electron ‘acquisition’ and ‘donation’
- This is why the first step of photosynthesis stores electrons on the C atom rather than on any other atom
- Because C can be stripped of its electrons a lot easier than N or S, the latter will just not give their electrons in an environment where C can do that for them first. As a result, and this is crucial for our field, the inorganic byproducts containing C, N, or S of the oxidation of organic matter in respiration (details in Chapter 5) are CO2, where carbon has been fully oxidized, and NH4+ and H2S, where N and S are still fully reduced, as they do not get oxidized during heterotrophic respiration.
- Notice that N and S only have variable numbers of electrons in inorganic molecules.
- The N atom of nitrite has two electrons for itself and is highly unstable in aerobic environments as oxygen will try to steal its electrons.
- Sulfur dioxide (SO2) and sulfite (SO32-) also are in very small quantities in the atmosphere (SO2) or in water as HSO3- (not represented in Table 2.1) and SO32-, as both will readily be oxidized in sulfuric acid and sulfate.
- Notice that N2 has 5 electrons for itself, which is quite a lot and, therefore, potentially is an important electron donor. However, the triple bond N≡N is very difficult to break and O2 does not, even at high temperatures. As a result, N≡N is an inert or unreactive gas, although it makes for about 78% of earth’s atmosphere. Nitrogen in N2 is thus referred to as unreactive nitrogen.
| Nb of e- stored on the atoms | C | N | S | P |
|---|---|---|---|---|
| 0 |
carbonate 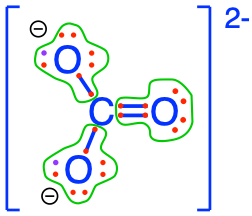
|
nitrate 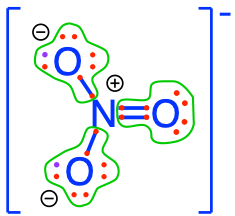
|
sulfate 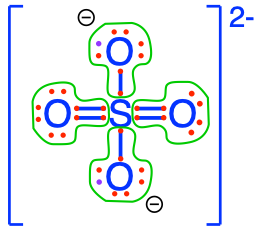
|
phosphate 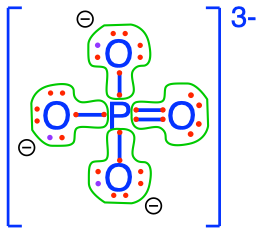
|
| 1 |
C#1 pyruvic acid 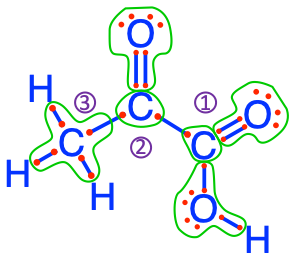
|
|||
| 2 |
C#2 pyruvic acid  carbon monoxide carbon monoxide 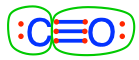
|
nitrite 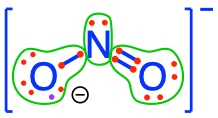
|
sulfite 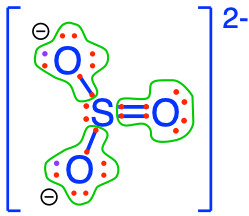 sulfur dioxide sulfur dioxide 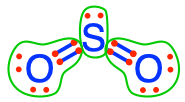
|
|
| 3 |
C#1 of glucose 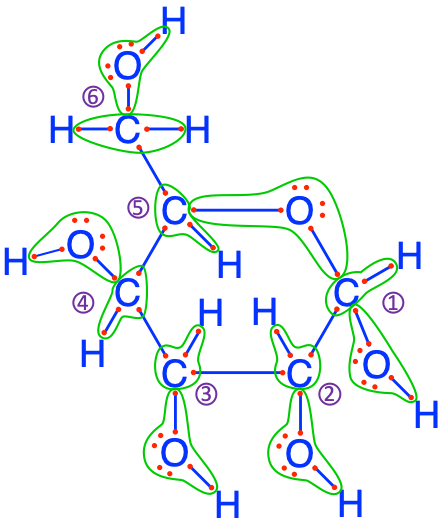
|
N#2 of nitrous oxide 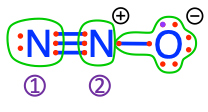
|
||
| 4 |
C#2 to C#5 of glucose 
|
|||
| 5 |
C#6 of glucose 
|
dinitrogen  nitrogen monoxide nitrogen monoxide 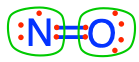 N#1 of nitrous oxide N#1 of nitrous oxide 
|
||
| 6 |
C of fatty acid 
|
|||
| 7 |
pyruvic acid (C#3) 
|
|||
| 8 |
methane 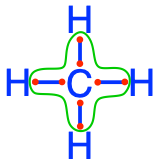
|
ammonium 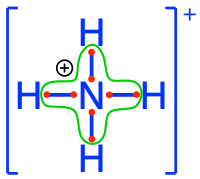 ammonia ammonia 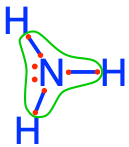 amine groups in amino-acids amine groups in amino-acids 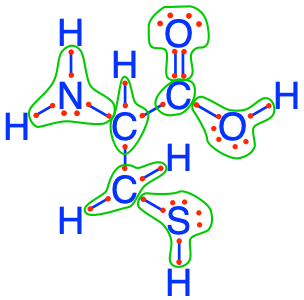
|
dihydrogen sulfide 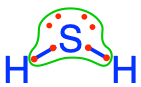 hydrogen sulfide hydrogen sulfide 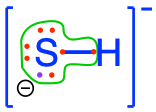 sulfide sulfide 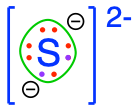 thiol groups in organic molecules thiol groups in organic molecules 
|
2.8 Examples of electrons stored on organic molecules
Not convinced, yet? Let us take the example of glucose. Glucose is the result molecule of photosynthesis, where electrons have been stored onto the carbon atoms, which originally contained none as the nutrient from which glucose molecule is assembled is CO2. How can one see this? In the equation (2.2), the half reactions for the production of glucose! Additional details on redox half-reactions are provided in the glossary. What is important to see here is that:
- 24 electrons are necessary to built glucose from CO2
- that these 24 electrons originally come from the oxygen atom of H2O
- that as oxygen atoms from water lose electrons, they form O2, where each atom has only 6 electrons for itself (Figure 2.4)
\[\begin{align} 6\,CO_2 + 24\,H^+ + 24\,e^- & \rightleftharpoons & C_6H_{12}O_6 + 6\,H_2O\\ 12\,H_2O & \rightleftharpoons & 6\,O_2 + 24\,H^+ + 24\,e^-\\ & &\\ \hline\\ 6\,CO_2 + 6\,H_2O & \to & C_6H_{12}O_6 + 6\,O_2 \tag{2.2} \end{align}\]
After what we have said about oxygen and its propensity to steal all electrons, why would it suddenly give electrons to C to form glucose? Indeed, there is some magic involved and it is called photosynthesis! For this to happen, magic catalysts must be able to steal the electrons from oxygen of H2O, and a lot of energy captured in the form of light is necessary!
And indeed in the end, thanks to the electron allocation concept, it is possible to visualize where all electrons on the glucose molecule are located as illustrated in Figure 2.8.

Figure 2.8: Electron allocation on the glucose molecule showing that the C atoms have 3 to 5, and an average of 4 electrons for themselves
So, hopefully you are now convinced thanks to both the equations and Figure 2.8 that indeed electrons are stored onto organic molecules! Notice that four of the C atoms have 4 electrons for themselves, while one has 3 and the last one has 5. On average though, each atom has 4. This actually is quite interesting as it corresponds to the number of electrons C has on its free form. This means that photosynthesis is not ‘trying’ to store more electrons than what it has in its free form: this is probably a lot more efficient than if it tried to store 6 like for the lipids (see Table 2.1 and Chapter 4), and yet provides an opportunity to store a lot of electrons.
Let us take another example with cysteine, which has N and S atoms in it in addition to C and O atoms. You can see in Figure 2.9 that the N and S atoms have 8 electrons for themselves, while the C atoms have 1, 4 and 5 electrons for themselves. Cysteine has thus stored a total of 28 electrons on a total of 5 CNS atoms or 40% more electrons per atom than glucose.

Figure 2.9: Electron allocation on the cysteine molecule showing the number of electrons stored on the C, N and S atoms
An alga who would use nitrate, sulfate and bicarbonates as its nutrients to anabolize cysteine, would need to find 28 electrons and energy to assemble cysteine, as no electrons come with the nutrients listed here. Interestingly, the electrons stored on N and S in cysteine are not useful as a source of electrons in the respiration chain of heterotrophic respiration. But the N and S atoms, which respectively appear as amine (-NH2) and thiol (-SH) radicals for cysteine in Figure 2.9, play a central role in molecular configuration . This is what the next chapter is going to show.
The last example is ATP (Adenosine TriPhosphate), illustrated in Figure 2.10. You can see that there are lots of red dots on the triphosphate side of the molecule, and see that phosphorus has zero electrons for itself…! Chapter 5 details the reasons how and why ATP carries energy. But here, one can see that surely, it is not thanks to electrons on P, since there are none! The triphosphate chain is bound to a ribose, itself bound to an adenine. Ribose + Adenine is called adenosine. On ribose, each carbon stores an average of 4 electrons, like all carbohydrates. There are lots of electrons stored on the 5 atoms of nitrogen on adenine (5 × 8 = 40). But these are not used for generating energy in the respiration chain. Instead, the large number of N atoms and electrons allow for electronic resonance on the adenine side, which makes it flat and very strong and very resistant to degradation. The number of electrons packed in a very resistant molecule must be put in relation with the high value of the ATP molecule.
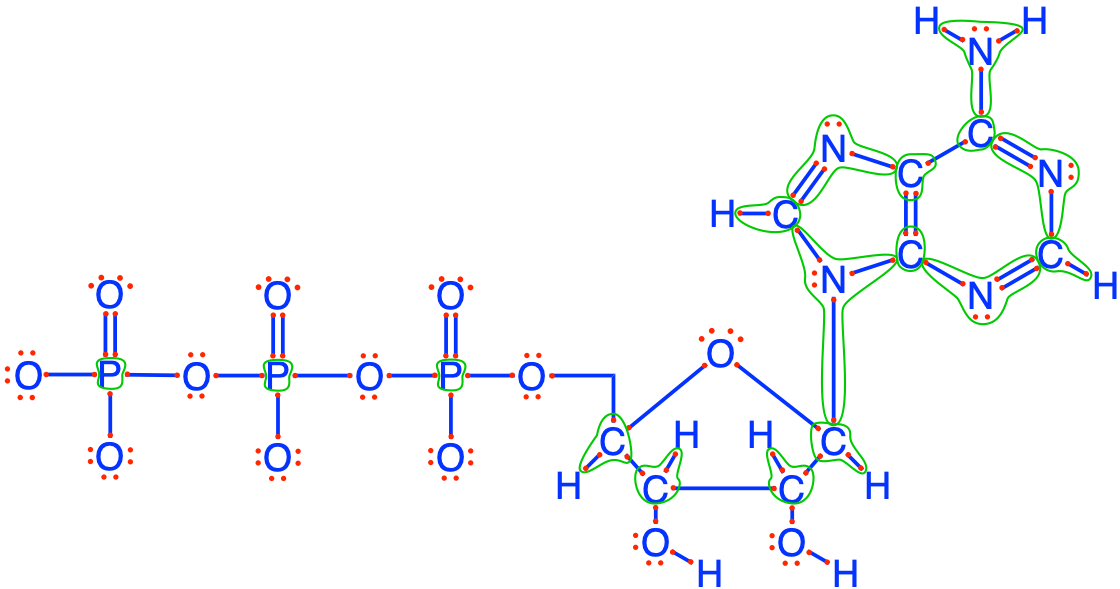
Figure 2.10: Electron allocation on the ATP molecule showing the number of electrons stored on the C (1 to 4), N (always 8), and P (always 0) atoms
Last but important point. It is possible that some of you are still wondering why the electrons on oxygen are not counted as the number of electrons stored on molecules, and particularly on organic molecules. Most oxygen atoms, outside of the O2 molecule, have already ‘stolen’ electrons from all other atoms, as we have now repeated quite a few times. So when these molecules are anabolized from molecules such as carbonate, nitrate, sulfate or phosphate, each oxygen atom is already loaded with 8 electrons. So, it cannot accept any additional ones. So each oxygen atom in organic and inorganic molecule, is not bringing any electron ‘to the table’. The only changes can occur on the C, N, and S atoms.
2.9 Summary on electron allocations
This concludes the chapter on electron allocation, and the first introduction to oxidation and reduction. We have learned that using the electron allocation rooted in the electronegativity concept, it is possible to visualize where the electrons are stored and realize that in the vast majority of the time:
- Oxygen ‘steals’ all electrons for itself and is fully reduced, unless it is in the form of O2, where it becomes very ‘hungry’ for electrons
- Hydrogen being less electronegative, always ‘gives’ its electron to CONS atoms
- Life’s secret to storing energy boils down to having high-energy electrons stored on C, N, and S atoms, although only the C atoms easily accept or donate electrons.
- Phosphorus almost always remains in the fully oxidized forms of phosphates (or polyphosphates), even when incorporated in organic molecules as we shall see in the next chapter. This is due to the fact that phosphorus’ electronegativity is lower than that of hydrogen. Phosphate plays a crucial role in energy conveyance, however, thanks to the diphospho anhydride bond.
The next step consists in presenting where and why organisms need N, S, and P, to build their structure. The next chapter presents the four molecular families with which all organisms, and therefore organic matter, are assembled.