Chapter 12 Applied thermochemistry: Oxido-Reduction equilibria
This chapter is still under construction
Chapter summary:
- A reducing agent, or reductant, is a molecule which can donate an electron e- and an oxidizing agent, or oxidant, base is an element that can accept an e-
12.0.1 The transfer of electrons in organotrophic denitrification
Much of the original intent of this book was started to clarify what happens during denitrification. We have seen in section 8.4 the general concept of denitrification. In organotrophic denitrification (denitrication can be lithothophic), it is the nitrogen atom of nitrate that accepts electrons from the carbon atoms of organic matter. In other words, the nitrate, under anaerobic conditions, oxidizes organic matter into \(CO_2\) and \(H_2O\), while the nitrogen atom is reduced into \(N_2\) and \(N_2O\), both of which are gases.
Let us make sure all this is clear. Again, let us use our favorite electron allocation tool to visualize where the electrons are, what atom can accept or donate electrons, and where electrons are transferred. In organotrophic respiration, the electrons are originally stored onto organic matter. Traditionally (e.g., Stumm and Morgan 1996) organic matter is simplified as \(CH_2O\) because only the carbon atoms participate in electron transfer, while the nitrogen and the sulfur atoms do not and stay reduced with 8 electrons for themselves. In organic chemistry, \(CH_2O\) corresponds to methanal, commonly called formalhehyde (Table 3.4). However, \(CH_2O\) in our case represents the ‘average’ behavior or carbon when it enters the respiratory chain (details in Chapter 5), and where 4 electrons are stored on the carbon. It turns out that in formaldehyde (\(CH_2O\)), carbon also has 4 electrons stored on carbon, so things are consistent. However, we prefer the representation of the ‘generic organic carbon’ as a secondary alcohol group (more properly expressed as \(-CHOH-\), although we will use \(CH_2O\) as a simplified and accepted notation), with 4 electrons on the carbon atom (Figure 12.1. On \(CH_2O\) the oxygen atom has 8 electrons for itself, but again, this energy is unavailalble because once oxygen has its 8 electrons, it will not donate them; details in Chapter 2). In other words, available electrons are the ones stored on the carbon atom of \(CH_2O\) in Figure 12.1.
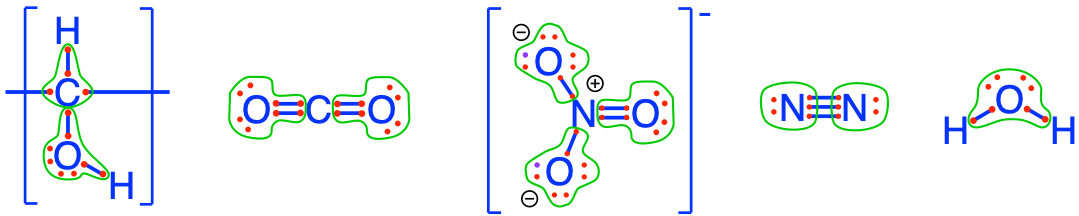
Figure 12.1: Electrons allocation on the carbon atoms showing 4 electrons stored on \(CH_2O\), surrogate for organic matter, and showing zero electrons on \(CO_2\); on the nitrogen atoms showing 0 electrons on \(NO_3^-\) and 5 electrons on \(N_2\); in all the molecules, including water, the oxygen always has 8 electrons for itself, and, being the most electronegative atom, does not participate in any electron transfer
So why, can nitrate accept electrons from organic matter? On the nitrate molecule, the oxygen atoms have stripped all the electrons from the nitrogen atom, and as a result, the nitrogen atom has zero electrons for itself (Figure 12.1). It can accept electrons, and only be an electron acceptor, and not a donor because it has nothing to give. Because the oxygen atoms on the nitrate molecules have all 8 electrons for themselves, cannot donate them or receive any additional ones, the oxygen atoms on nitrate DO NOT participate in the transfer of electrons during denitrification.
On a redox reaction side, the reduction of nitrate into dinitrogen involves the \(NO_3^-/N_2\) redox couple and can be written as:
\[\begin{equation} 2NO_3^- + 12 H^+ + 10 e^- \rightleftharpoons N_2 + 6H_2O \tag{12.1} \end{equation}\]
One can see in reaction (12.1) that the oxygen atoms that were on the nitrate are just moved to water. The oxygen atoms either on the nitrate or the water molecules have 8 electrons for themselves (Figure 12.1). There has thus been no electron transfer to or from the oxygen atoms, regardless of the reactants or products of this reaction. It is important to emphasize again that oxygen atoms on nitrate DO NOT participate in the transfer of electrons in denitrification, or in any other anaerobic respiratory processes. Where did the electrons go, then? They went on the nitrogen atom. And one can see that on the dinitrogen molecule (Figure 12.1), each nitrogen atom now has 5 electrons for itself. This is why in reaction (12.1), there are two molecules of nitrate as reactants, yielding one molecule of \(N_2\) for a total of 2×5 = 10 electrons transferred. Because the nitrogen atom has accepted electrons, we say that it has be reduced. Because the byproduct of this reduction is a gas which escapes to the environment, and is not assimilated by the denitrifier microbes that do the reduction, we say that denitrification is a dissimilatory reduction .
There is no such thing as ‘floating electrons’ in solution. So the reduction of the nitrogen atom of nitrate has to be associated with an oxidation, in the case of the organotrophic denitrification, to the oxidation of organic matter. This involves the redox couple \(CO_2/CH_2O\). The oxidation of organic matter (\(CH_2O\) for us in this example as detailed above) results in the loss of four electrons originally stored on the carbon of \(CH_2O\), resulting in \(CO_2\) where the carbon atom has lost all its electrons (Figure 12.1).
Redox half-reactions are always written with the oxidant on the left and the reductant on the right. As a result, instead of expressing the oxidation of \(CH_2O\) into \(CO_2\), it is the opposite reaction that is written by convention in (12.2), i.e., the reduction of \(CO_2\) into \(CH_2O\) (which incidentally corresponds to what happens during photosynthesis):
\[\begin{equation} CO_2 + 4 H^+ + 4 e^- \rightleftharpoons CH_2O + H_2O \tag{12.2} \end{equation}\]
The overall organotrophic denitrification is summarized in reaction (12.3) by multiplying the first half-reaction (12.1) by a factor of 2 and the second (12.2) by a factor of 5 to balance the number of electrons transferred.
\[\begin{gather*} 4NO_3^- + 24 H^+ + 20 e^- \rightleftharpoons 2N_2 + 12H_2O \\ 5CO_2 + 20 H^+ + 20 e^- \rightleftharpoons 5CH_2O + 5H_2O \\ \hline 5CH_2O + 4NO_3^- + 4 H^+ \rightleftharpoons 5CO_2 + 2N_2 + 7H_2O \tag{12.3} \end{gather*}\]
Using reaction (12.3) and the electron allocation of the reactants and products as illustrated in Figure 12.1, one might see that oxygen is not involved in any step of the transfer of electrons in denitrification. This might be somewhat surprising to the reader, and it is not impossible that one might have read the contrary in some publications and books. It is tempting to treat oxygen atoms the same, whether they be in the form of \(O_2\) or \(NO_3^-\). But we have said times and again in this book that this is a big trap not to fall into! Oxygen is the most electronegative atom, and as a result, it tends to ‘steal’ electrons for itself from atoms to which it is bonded. Consequently, oxygen atoms bonded to CHNSP atoms always have 8 electrons for themselves. The only times when oxygen atoms do not have 8 electrons for themselves is when oxygen is bonded to itself, at least partly. This corresponds to dioxygen 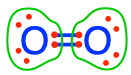 where each atom has 6 electrons for itself, and to hydrogen peroxide
where each atom has 6 electrons for itself, and to hydrogen peroxide 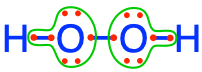 where each oxygen atom has 7 electrons for itself. The fact that the most electronegative atom, i.e., oxygen, existing in the form of \(O_2\) on our planet, drives most of the electron transfers! Indeed, oxygen ‘badly wants’ 8 electrons for itself. And in aerobic respirations, it manages just that.
where each oxygen atom has 7 electrons for itself. The fact that the most electronegative atom, i.e., oxygen, existing in the form of \(O_2\) on our planet, drives most of the electron transfers! Indeed, oxygen ‘badly wants’ 8 electrons for itself. And in aerobic respirations, it manages just that.
In Figure 12.2 the electron transfer scheme introduced in Chapter 5 is combined with the half-reactions (12.1) and (12.2), and with the electron allocation diagrams. The additional piece of information in Figure 12.2 is the fate of the N, S, and P atoms originally part of the organic matter during its oxidation. In organotrophic respirations, the byproducts of the oxidation of organic matter are \(CO_2\), \(NH_4^+\), \(H_2S\), and \(PO_4^{3-}\), because, again, only the carbon atoms participate in the transfer of electrons.
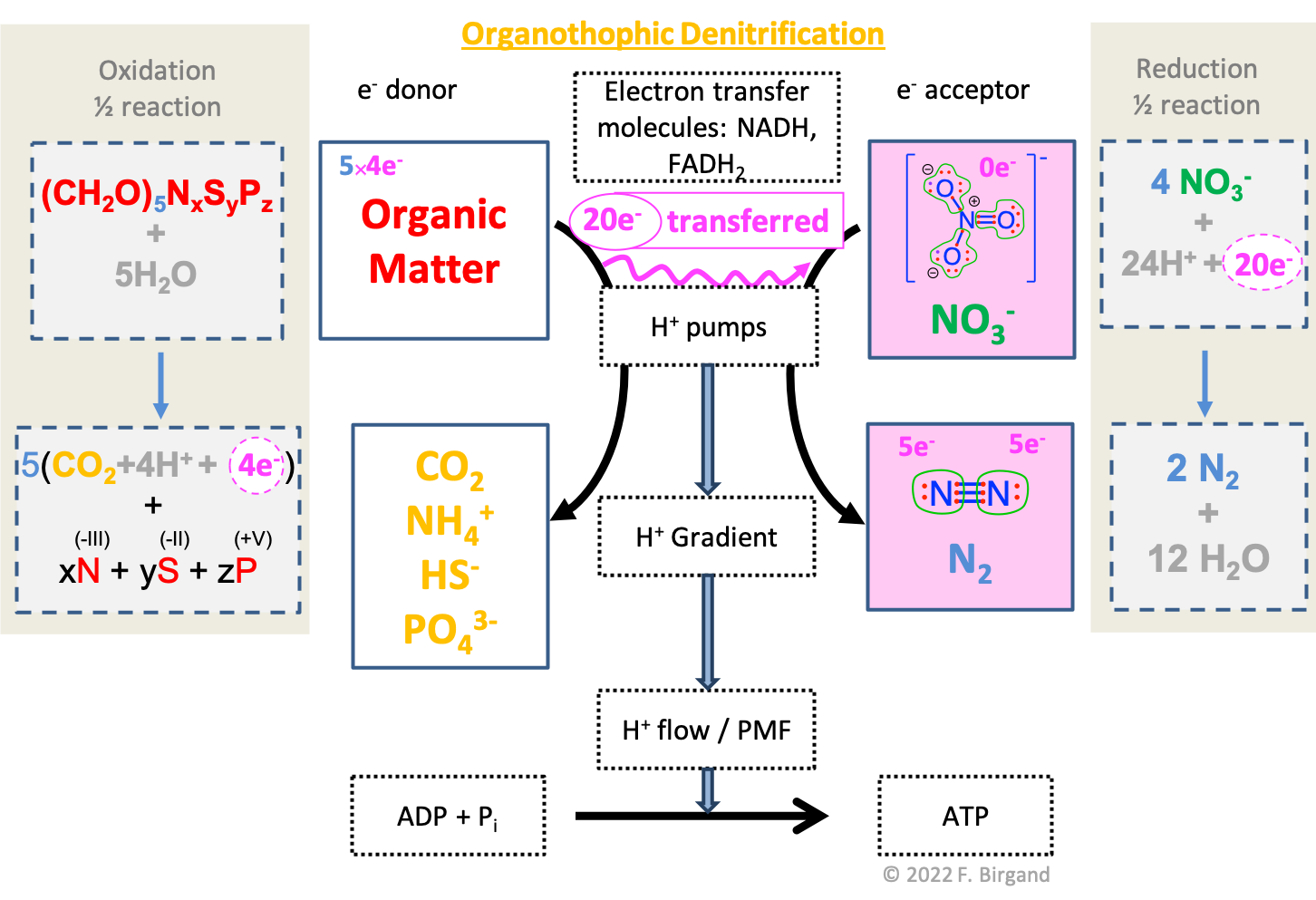
Figure 12.2: Combined electron transfer scheme with the electron allocation, the transfer of electrons, and the corresponding half redox reactions for organotrophic denitrification
12.0.2 Some outside the box redox reactions
12.0.2.1 Guanidinium nitrate reaction to inflate air bags
Guanidinium nitrate  , commonly called guanidine nitrate, is a crystal that contains together potential electron donors in the form of fully reduced nitrogen on the guanidinium cation and fully oxidized nitrogen on the nitrate anion side. This crystal is used in air bags of vehicles as it is rather stable, only needs a spark to explode, and has a relatively low temperature of reaction. Let us explore this reaction. First, the hint that it is used to rapidly blow air bags tells us that gas(es) must be produced. Because the nitrogen atoms of the guanidinium side have 8 electrons to give, and nitrate ions have none and can gain some, the end gas product that contains nitrogen must have a number of electrons somewhat between the two. The first guess is \(N_2\) as each atom has 5 electrons for itself. So the two redox couples at play must be \(NO_3^-/N_2\) and \(N_2/C(NH_2)_3^+\).
, commonly called guanidine nitrate, is a crystal that contains together potential electron donors in the form of fully reduced nitrogen on the guanidinium cation and fully oxidized nitrogen on the nitrate anion side. This crystal is used in air bags of vehicles as it is rather stable, only needs a spark to explode, and has a relatively low temperature of reaction. Let us explore this reaction. First, the hint that it is used to rapidly blow air bags tells us that gas(es) must be produced. Because the nitrogen atoms of the guanidinium side have 8 electrons to give, and nitrate ions have none and can gain some, the end gas product that contains nitrogen must have a number of electrons somewhat between the two. The first guess is \(N_2\) as each atom has 5 electrons for itself. So the two redox couples at play must be \(NO_3^-/N_2\) and \(N_2/C(NH_2)_3^+\).
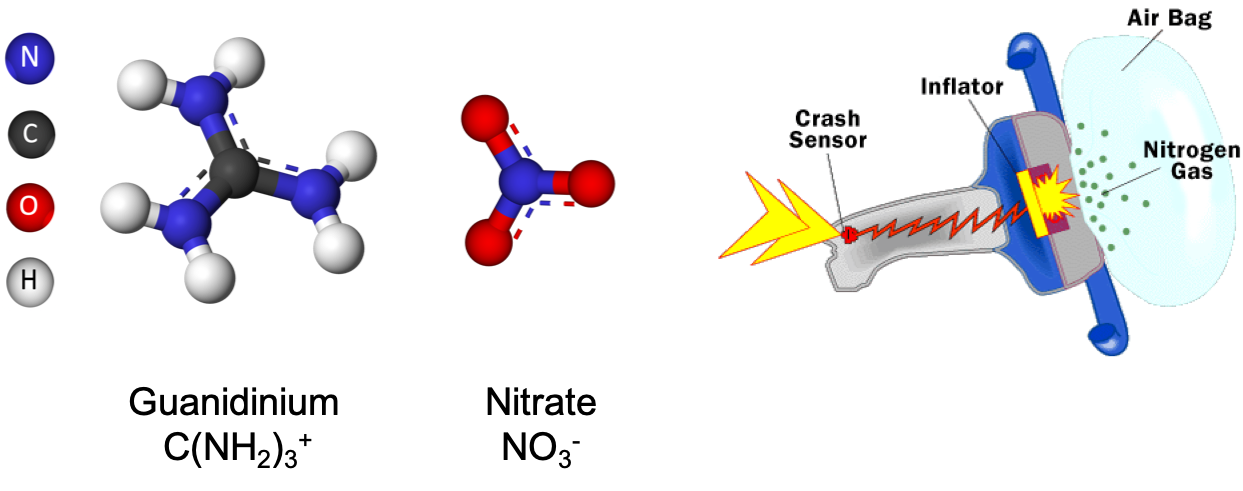
Figure 12.3: Ball and stick structure of guanidinium nitrate sometimes used as a fuel for airbag inflation
However, on one guanidinium, there are three nitrogen atoms fully reduced for a combined 24 electrons to give (8 e- on each of the N atom), although when oxidized into \(N_2\) (5 e- on each of the N atom), each atom would lose 3 electrons for a combined 9 electrons lost. Nitrate reduction into \(N_2\) involves accepting 5 electrons. So there is an imbalance of 4 electrons in the electron transfer from nitrogen atoms of guanidinium to the one nitrogen of nitrate… The only solution is for the carbon of guanidinium, which has no electron for itself, to accept these 4 electrons. But then, how can carbon atoms be floating around on their own…? The octet rule must be fulfilled in all cases… Carbon atoms can actually form direct bonds with themselves as solid carbon, written as \(C_{(s)}\), and therefore accept 4 electrons. If dioxygen is present, then the formation of solid carbon usually does not happen as dioxygen steals these electrons first. But in the case of an airbag cartridge, there is very little space for dioxygen, the formation of \(C_{(s)}\) can occur.
Now, one can balance the two half reactions involved. Nitrate being the most oxidizing agent, the first redox couple is considered. Its first reaction can be written as:
\[\begin{equation} 2NO_3^- + 12 H^+ + 10 e^- \rightleftharpoons N_2 + 6H_2O \tag{12.1} \end{equation}\]
The dinitrogen/guanidinium redox couple is quite interesting as it involves another redox reactive atom: carbon. In these cases, the general approach is to leave the atoms as they are, provided that there is a possibility for the octet rule of the reactants/products to be fulfilled. Here, the solution is solid carbon \(C_{(s)}\). Hence the half redox equation for this couple:
\[\begin{equation} 3N_2 + 2C_{(s)} + 12 H^+ + 10 e^- \rightleftharpoons 2C(NH_2)_3^+ \tag{12.4} \end{equation}\]
On both half-reactions (12.1) and (12.4), the same number of electrons (10) are transferred and can be directly combined to obtain the final reaction (12.5).
\[\begin{gather*} 2NO_3^- + 12 H^+ + 10 e^- \rightleftharpoons N_2 + 6H_2O \\ 3N_2 + 2C_{(s)} + 12 H^+ + 10 e^- \rightleftharpoons 2C(NH_2)_3^+ \\ \hline C(NH_2)_3^+ \space NO_3^- \rightleftharpoons 2N_2 + 3H_2O + C_{(s)} \tag{12.5} \end{gather*}\]
The decomposition of one mole of solid guanidinium nitrate, upon an activation energy provided by a spark, produces into 5 moles of gas (2 moles of dinitrogen and 3 moles of water), and one mole of solid carbon. Because the electron donors and acceptors are closely packed together, the reaction is not limited by the transport of an electron acceptor near the donor (as is the case for burning wood for example), this reaction is fast. An instantaneous reaction that produce 5 moles of gas from a mole of solid compound has an explosive effect, hence its use in air bag deployment.
Now, one might ask: why is it that the only hypothetical gas produced should be \(N_2\) and not \(N_2O\) for example? After all, in nitrous oxide  , the middle oxygen has 3 electrons for itself and the other one 5, for an average of 4, which is closer to the midpoint between the amine groups (each N contains 8) and the nitrate (0 electrons on N). Let us thus explore the \(NO_3^-/N_2O\) and \(N_2O/C(NH_2)_3^+\) redox couples and the resulting reaction.
, the middle oxygen has 3 electrons for itself and the other one 5, for an average of 4, which is closer to the midpoint between the amine groups (each N contains 8) and the nitrate (0 electrons on N). Let us thus explore the \(NO_3^-/N_2O\) and \(N_2O/C(NH_2)_3^+\) redox couples and the resulting reaction.
The half-reaction of the most oxidizing redox couple \(NO_3^-/N_2O\) can be written as:
\[\begin{equation} 2NO_3^- + 10 H^+ + 8 e^- \rightleftharpoons N_2O + 5H_2O \tag{12.6} \end{equation}\]
And the half-reaction of the more reducing \(N_2O/C(NH_2)_3^+\) can be written as:
\[\begin{equation} 3N_2O + 2C_{(s)} + 18 H^+ + 16 e^- \rightleftharpoons 2C(NH_2)_3^+ + 3H_2O \tag{12.7} \end{equation}\]
Half-reaction (12.6) needs to be multiplied by a factor of 2 to have the same number of electrons transferred (16). The resulting oxidation of guanidinium nitrate leading to the formation of nitrous oxide can be written as in (12.8).
\[\begin{gather*} 4NO_3^- + 20 H^+ + 16 e^- \rightleftharpoons N_2O + 10H_2O \\ 3N_2O + 2C_{(s)} + 18 H^+ + 16 e^- \rightleftharpoons 2C(NH_2)_3^+ + 3H_2O \\ \hline 2C(NH_2)_3^+ + 4NO_3^- +2H^+ \rightleftharpoons 5N_2O + 2C_{(s)} + 7H_2O \tag{12.8} \end{gather*}\]
Clearly this reaction is possible in theory. However, in practice it probably does not happen because 1) it requires the presence of protons, which would not be there in the guanidinium nitrate crystals, and 2) it would require twice as many nitrate as many guanidinium, and here too, this stochiometry is not that of the guanidinium nitrate salt. So this reaction is probably not happening when airbags are set on.
12.0.2.2 Chemolithotrophic denitrification on pyrite
Pyrite (\(FeS_2\)) is an interesting mineral and illustrates quite well the difference between covalent bonds and an ionic bond. In the case of covalent bonds, electrons are shared between non-metal ions, in ionic bonds, one metal and a non-metal atom exchange electrons. In ionic bonds, we cannot use the electronegativity concept any longer to allocate electrons. So, although Sulfur atom is more electronegative (2.58) than iron (1.83), Sulfur does not steal electrons from iron. So the ions involved in pyrite are \(Fe^{2+}(S^-)_2\). Iron is thus in its most reduced form, for an oxidation state (OS) of (+II) corresponding to its charge referred to as ferrous iron or iron (II), and sulfur is also reduced in the form of \(S^-\), having 7 electrons for itself for an oxidation state of (-I). The summation of the OS is +II -I -I = 0, corresponding to the zero charge of the overall crystal.
Because the sulfur has 7 electron for itself, it can be oxidized, obviously by dioxygen, but interestingly for biogeochemical purposes, by nitrate as well. The full oxidation of sulfur, i.e., the loss of all 7 electrons, leads to the formation of sulfate. The reduction of nitrate generally leads to the formation of dinitrogen although the reduction all the way to ammonium is possible in the reaction called Dissimilatory Reduction of Nitrate into Ammonium (DNRA). In nature, it seems that the reduction of nitrate stops at the \(N_2\) stage.
From all this information, it is possible to draw the two redox couple involved. The more oxidative couple is \(NO_3^-/N_2\) and the one that involves pyrite oxidation into sulfate is \(SO_4^{2-}/FeS_2\).
The nitrate reduction into dinitrogen is half-reaction (12.1),
\[\begin{equation} 2NO_3^- + 12 H^+ + 10 e^- \rightleftharpoons N_2 + 6H_2O \tag{12.1} \end{equation}\]
and the pyrite oxidation:
\[\begin{equation} 2SO_4^{2-} + Fe^{2+} + 16 H^+ + 14 e^- \rightleftharpoons FeS_2 + 8 H_2O \tag{12.9} \end{equation}\]
The overall reduction of nitrate by pyrite is summarized in reaction (12.10) by multiplying the first half-reaction (12.1) by a factor of 7 and the second (12.9) by a factor of 5 to balance the electrons transferred.
\[\begin{gather*} 14NO_3^- + 84 H^+ + 70 e^- \rightleftharpoons 7N_2 + 42H_2O \\ 10SO_4^{2-} + 5Fe^{2+} + 80 H^+ + 70 e^- \rightleftharpoons 5FeS_2 + 40 H_2O \\ \hline 5FeS_2 + 14NO_3^- + 4H^+ \rightleftharpoons 10SO_4^{2-} + 5Fe^{2+} + 7N_2 + 2H_2O \tag{12.10} \end{gather*}\]
Now, there is another twist to chemolithotrophic denitrification using pyrite as the primary electron acceptor. Several studies surmised that nitrate could be further denitrified by the produced \(Fe^{2+}\) of pyrite oxidation (Kölle, Strebel, and Böttcher 1983, 1985, 1990; Pauwels 1994, 1994; Somlette 1999). But it was not until 1996, that actual evidence of Nitrate-Dependent Fe-Oxidation (NDFO) or Nitrate Reducing Fe Oxidation (NRFeOx, K. L. Straub et al. (1996)) microbial activity was brought forth. In other words, people have found that there were bacteria able to use ferrous Fe (II) as an electron donor, and nitrate as the electron acceptor. This has later been confirmed in many different environments including lake sediments, in pyrite containing schist, sandy and gravelly sediments, etc. (K. L. Straub et al. 1996; K. L. Straub and Buchholz-Cleven 1998; Lack et al. 2002; Kristina L. Straub et al. 2004; Kappler and Straub 2005; Kappler, Schink, and Newman 2005; Weber, Achenbach, and Coates 2006; Muehe et al. 2009; Blöthe and Roden 2009; Chakraborty et al. 2011; Chakraborty and Picardal 2013; Melton et al. 2014; Liu et al. 2019; reviewed by Bryce et al. 2018) (Figure 12.4).
![I. Chemolithotrophic bacteria *Thiobacillus denitrificans* used pyrite (FeS~2~) as electron donor, and nitrate as electron acceptor, reducing them to N~2~ and N~2~O. II. The ferrous iron Fe^2+^ produced is then used by other microorganisms that use Fe^2+^ as electron donors and nitrate, again as electron acceptor. These are called Nitrate Reducing Fe Oxidazing organisms. Some are autotrophs, mixotrophs (need both organic carbon and Fe^2+^ to live), or chemodenitrifiers (require organic carbon and have no enzymatic component of Fe(II) oxidation). After [@Bryce2018-cb]](pictures/pyrite-NRFeOx.png)
Figure 12.4: I. Chemolithotrophic bacteria Thiobacillus denitrificans used pyrite (FeS2) as electron donor, and nitrate as electron acceptor, reducing them to N2 and N2O. II. The ferrous iron Fe2+ produced is then used by other microorganisms that use Fe2+ as electron donors and nitrate, again as electron acceptor. These are called Nitrate Reducing Fe Oxidazing organisms. Some are autotrophs, mixotrophs (need both organic carbon and Fe2+ to live), or chemodenitrifiers (require organic carbon and have no enzymatic component of Fe(II) oxidation). After (Bryce et al. 2018)
As a result, reaction (12.10) actually continues as the produced ferrous Fe (II) can be further oxidized by nitrate. The most oxidizing couple is still \(NO_3^-/N_2\), and the most reducing is now \(Fe(OH)_3/Fe^{2+}\). One could choose \(Fe^{3+}\) instead of \(Fe(OH)_3\), but at groundwater pH and reduced conditions when nitrate is still present, Fe (III) is more stable as iron hydroxides (Hem 1985; Stumm and Morgan 1996; Fetter 2008; Torrentó et al. 2010; reviewed by Bryce et al. 2018). Other authors proposed that the goethite mineral (\(FeOOH\)) would be formed instead of \(Fe(OH)_3\) iron hydroxides (Kölle, Strebel, and Böttcher 1985, 1990; Cheng et al. 2020). Ottley et al. (1997) actually listed 12 other possible \(Fe^{2+}\) oxidation reactions by nitrate. The one described below appears to be considered as prevalent (reviewed by Bryce et al. 2018).
The half reaction for the \(Fe(OH)_3/Fe^{2+}\) can be written as:
\[\begin{equation} Fe(OH)_3 + 3 H^+ + 1 e^- \rightleftharpoons Fe_2^+ + 3H_2O \tag{12.11} \end{equation}\]
Combining both half-reactions (12.1) and (12.11) and multiplying each by the proper factor to exchange the same number of electrons yields:
\[\begin{gather*} 2NO_3^- + 12 H^+ + 10 e^- \rightleftharpoons N_2 + 6H_2O \\ 10Fe(OH)_3 + 30 H^+ + 10 e^- \rightleftharpoons 10Fe_2^+ + 30H_2O \\ \hline 5Fe^{2+} + NO_3^- + 12H_2O \rightleftharpoons 5Fe(OH)_3 + 9H^ + \frac{1}{2}N_2 \tag{12.12} \end{gather*}\]
Now, literally adding reactions (12.10) and (12.12) yields:
\[\begin{gather*} 5FeS_2 + 14NO_3^- + 4H^+ \rightleftharpoons 10SO_4^{2-} + 5Fe^{2+} + 7N_2 + 2H_2O \\ 5Fe^{2+} + NO_3^- + 12H_2O \rightleftharpoons 5Fe(OH)_3 + 9H^+ + \frac{1}{2}N_2 \\ \hline 5FeS_2 + 15NO_3^- + 10H_2O \rightleftharpoons 5Fe(OH)_3 + \frac{15}{2}N_2 + 10SO_4^{2-} + 5H^+ \tag{12.13} \end{gather*}\]
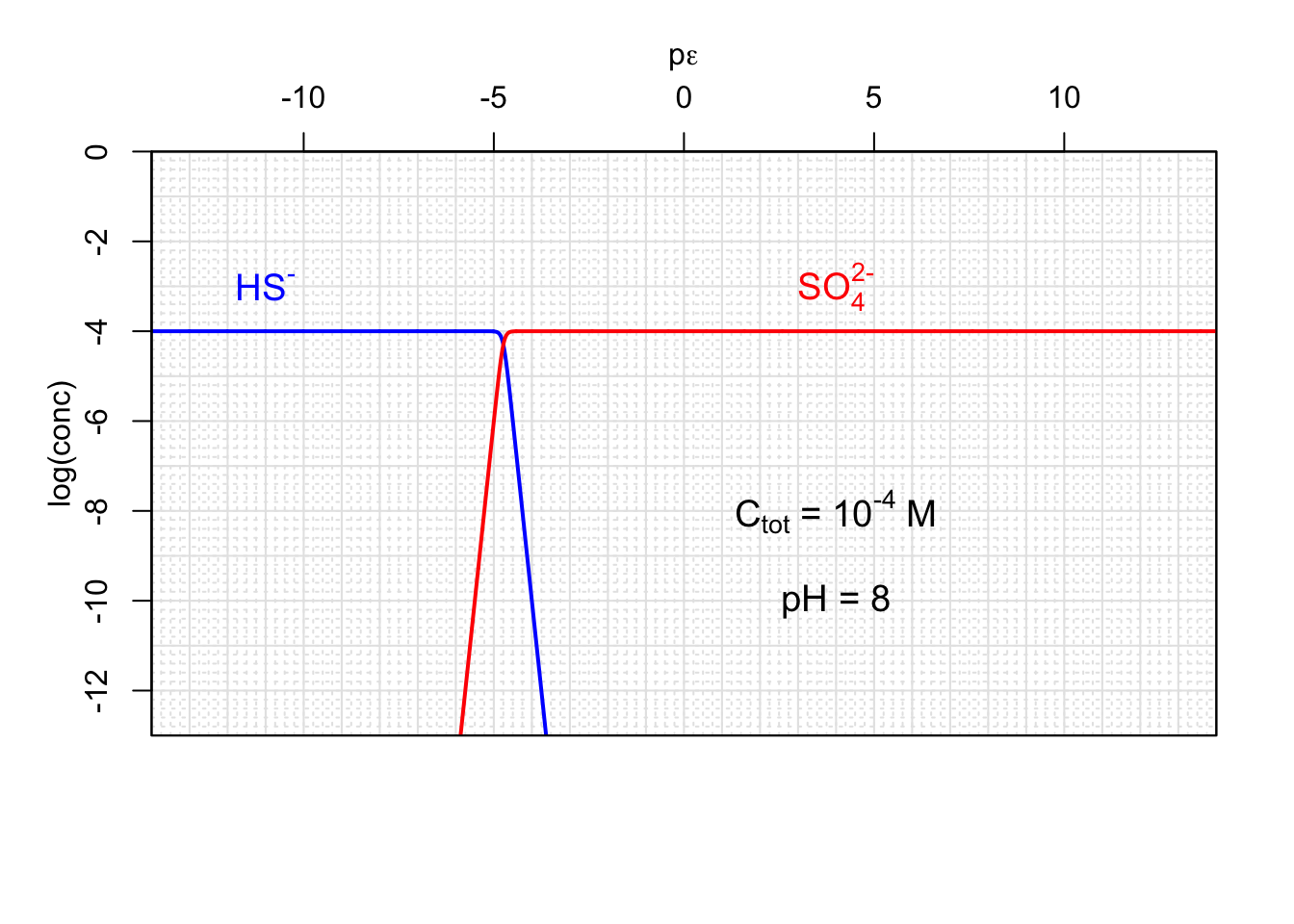
Figure 12.5: Concentrations of Sulfate and hydrogen sulfide as a function of pε
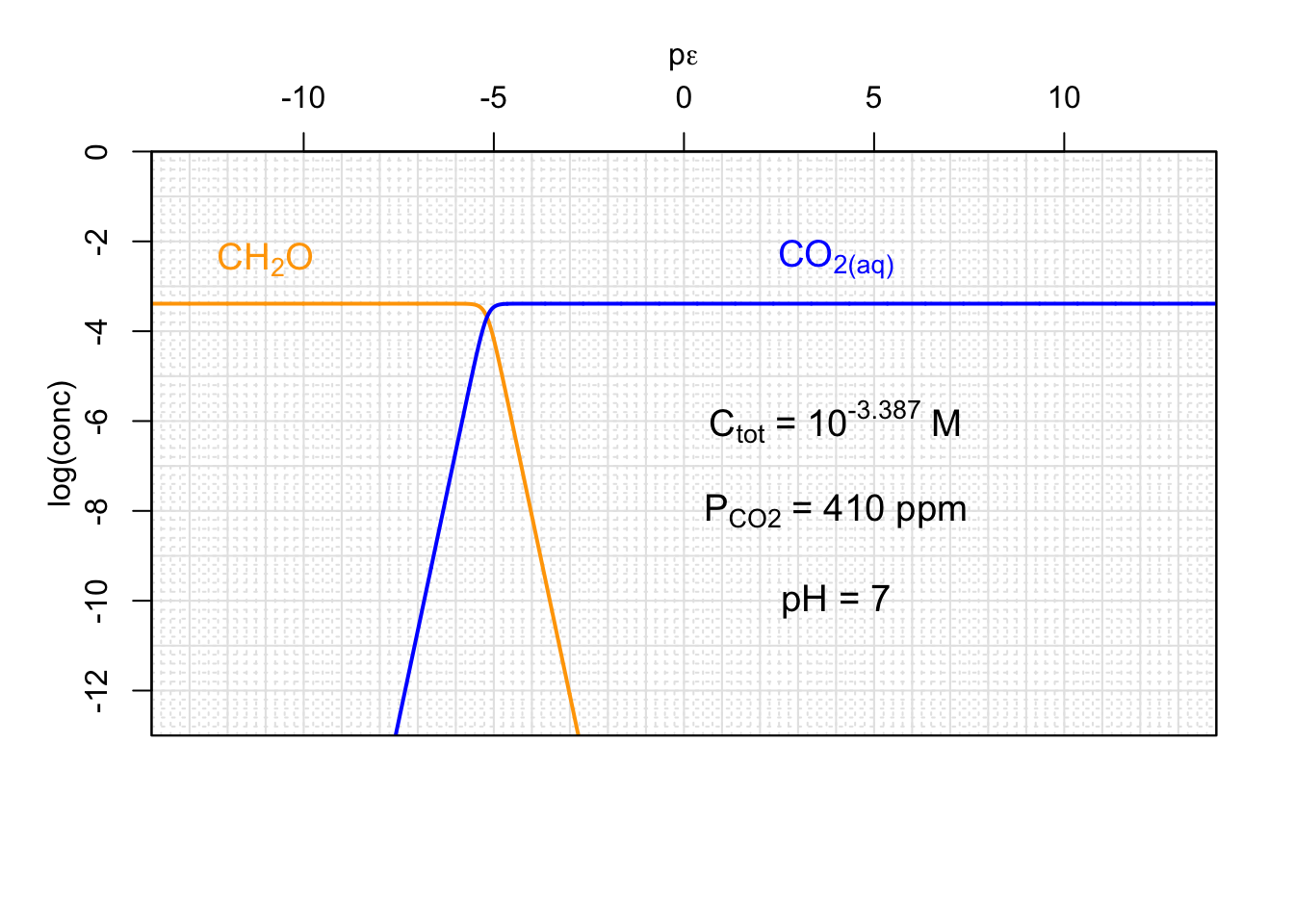
(#fig:CO2-CH_2O-pe)Concentrations of a generic carbohydrate CH_2O and CO2 that would be in equilibrium with the atmosphere as a function of pε and for a given pH
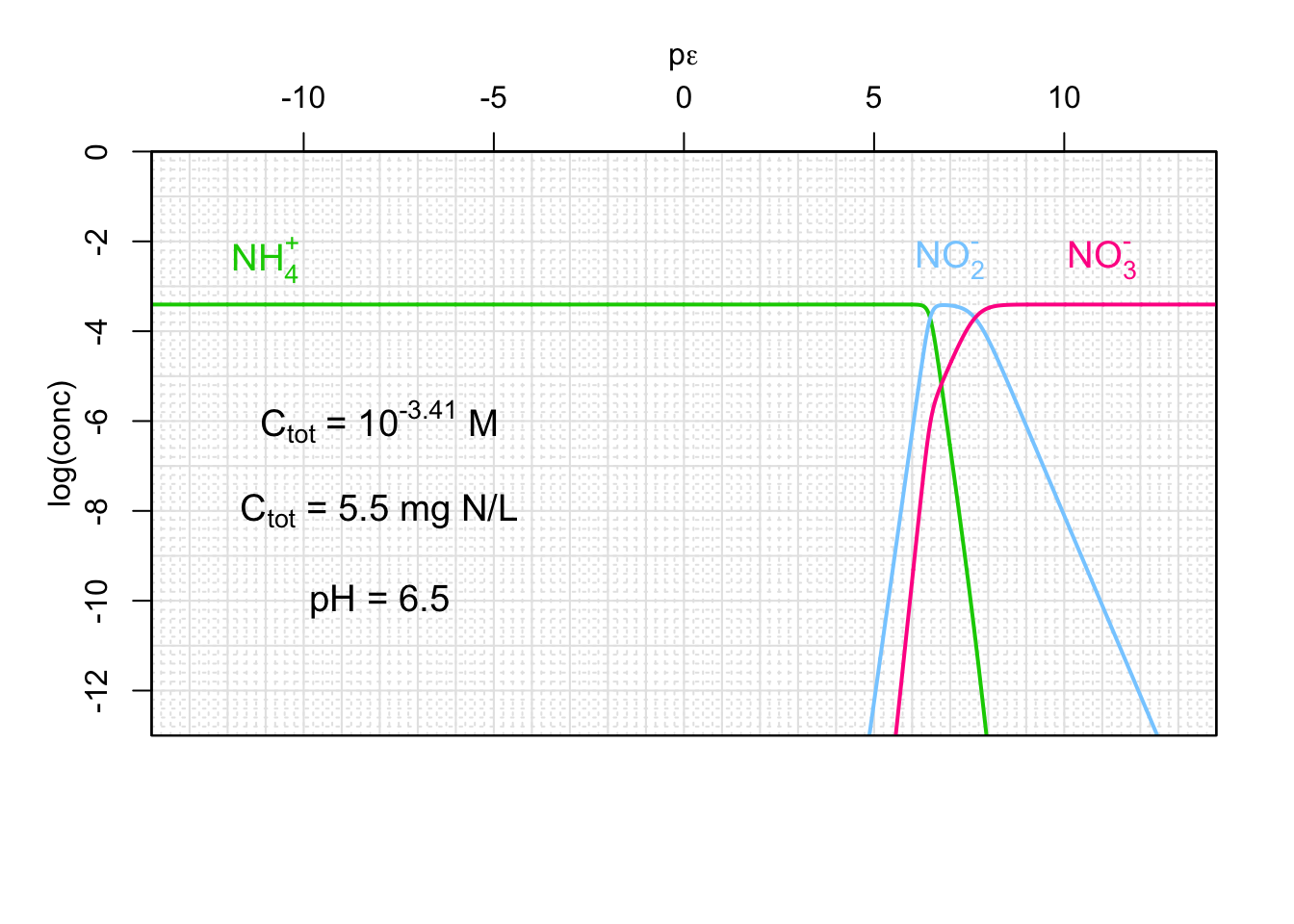
Figure 12.6: Concentrations ammonium, nitrite, and nitrate as a function of pε and for a given pH
This chapter is still under construction